ALABAMA, USA — The FDA and CDC have pressed pause on the use of the J&J vaccine, but why?
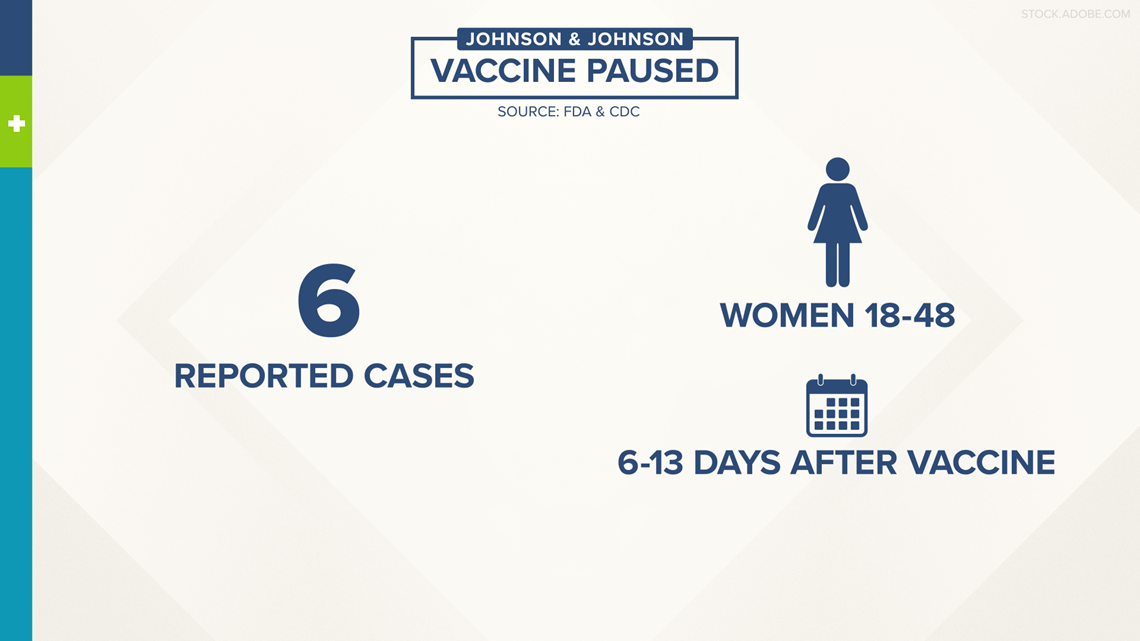
"Together the CDC and the FDA are reviewing data involving 6 reports of a rare type of blood clot called Cerebral Venous Sinus Thrombosis or CVST in combination with low levels of platelets in the blood called Thrombocytopenia - in women ages 18 to 48 who presented with symptoms between 6 and 13 days after receiving the Johnson & Johnson or Janssen COVID-19 vaccine," said Director of the FDA's Center for Biologics, Evaluation and Research, Peter Marks, M.D., Ph.D.

This type of blood clot is extremely rare, so treatment is also different from typical blood clots, which concerns health care workers.
"The FDA will revise the fact sheet for the health care providers administering vaccine and the fact sheet for recipients and caregivers for the Janssen COVID-19 vaccine to include this adverse event information to ensure health care providers are able to make appropriate benefit-risk determinations for their patients," said Marks.
Of the six clot cases seen in the United States, one patient died and another is in critical condition, but the risk of this happening to most recipients lowers as time passes.
"For people who recently got the vaccine within the last couple of weeks, they should be aware to look for any symptoms. If you've received the vaccine and have developed severe headaches, abdominal pain, leg pain or shortness of breath, you should contact your health care provider and seek medical treatment," said Principal Deputy Director of the CDC, Anne Schuchat, M.D.
This is national news but what does it mean for the Tennessee Valley? Well, Governor Ivey released a statement similar to the FDA and CDC.


Highlighted: "It is important to know that the adverse effects potentially stemming from the Johnson & Johnson shot have been extremely rare in the country, but out of an abundance of caution, Alabama is temporarily pausing these shots until we know more," said Ivey.
Ivey says she commends Dr. Harris from the Alabama Department of Public Health for taking this step in our state. Dr. Harris says this hiccup will not stop vaccination efforts in Alabama.
"In terms of our total number of doses you know, it doesn't really affect that much and we've given you know, 71,000 doses out of you know around 2.1 million in total shots so far, so it hasn't represented a large number of our vaccines," said State Health Officer of the Alabama Department of Public Health, Scott Harris, M.D.

